Artificial vision is nowadays a fast-developing medical field able to restore a certain degree of vision functionality, and hence self-sufficiency, in selected patients who lost their vision in adult life.
Vision starts when light coming from the surrounding environment hits the retina and photoreceptors transform it into electrical stimuli. The process is then completed when the electrical stimuli are passed to other retinal cells and, via the optic nerve, to the area of the brain cortex that processes them into images.
Many pathologies and traumas can impede the conversion of light into electrical stimuli or the passage of the latter to the brain, hence hindering vision. Artificial vision comes in aid when one of the elements that take part in the visual process is lost or damaged, and depending on which the lost element is, artificial vision can act in two main different ways.
When vision is prevented by loss or damage of photoreceptors, for example due to a pathology of the retina such as retinitis pigmentosa (RP) or age-related macular degeneration (AMD), artificial vision comes in aid by restoring the function that triggers the visual process: sensing light and transform it into electrical stimuli.
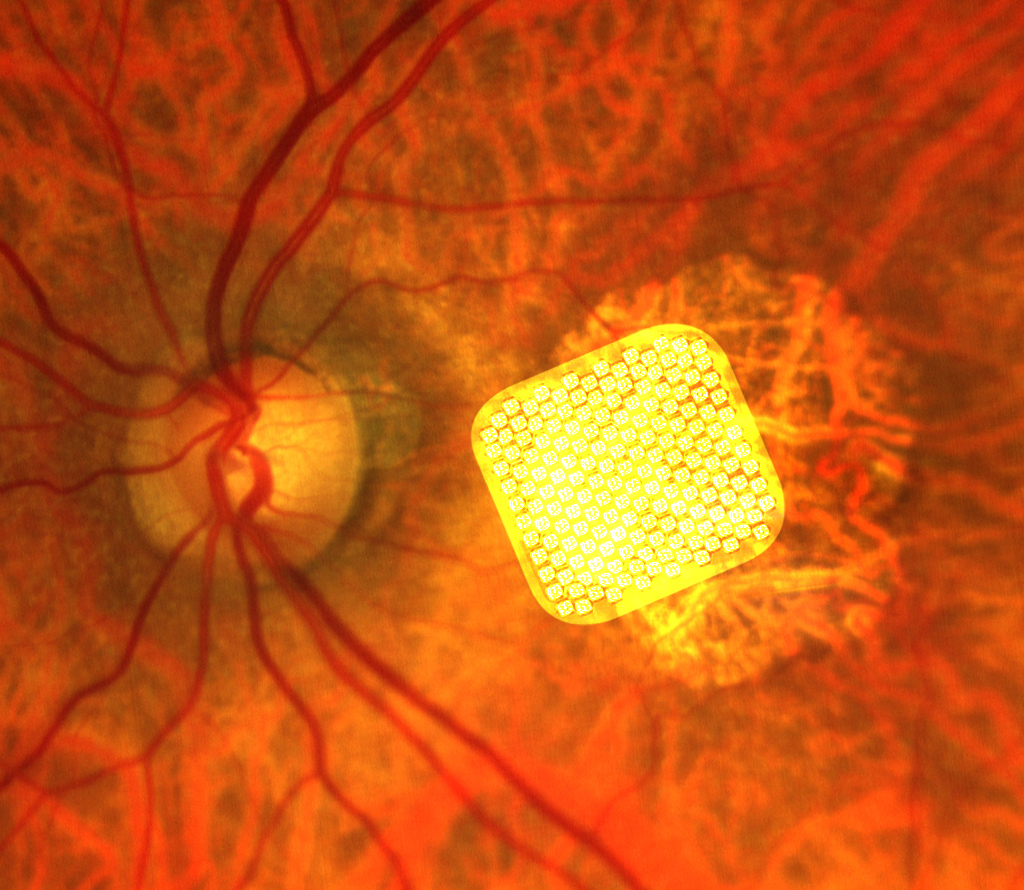
This is done by implanting a medical device known as retinal prosthesis or retinal microchip inside the eye, in strict contact with the retina. A retinal prosthesis is able to sense the light that hits the retina and give rise to electrical stimuli similarly to what photoreceptors do. Retinal prostheses receive information by a miniaturized video camera mounted on glasses and generate electrical stimuli that follow a simplified geometrical pattern of the “seen object”. The electrical stimuli are naturally transmitted to the brain, where they are finally perceived as a pattern of flashes of light (phosphenes). The different patterns of phosphenes reflect the shapes of the seen objects and can be recognized by the patients thanks to a specific vision training process.
The first retinal prosthesis to obtain the CE mark in Europe (in 2011) and the FDA approval in the USA (in 2013) was the Argus. Since then, significant advances have been achieved in the field and new retinal prostheses have become much easier to implant and allow a more detailed visual perception. Several clinical trials have demonstrated the safety and efficacy of retinal prostheses and one of the most recent models, PRIMA, has been and continues to be tested in AMD patients with excellent results and more trials are now ongoing also in patients with RP.